PharmaShots Weekly Snapshots (May 27 – May 31, 2024)
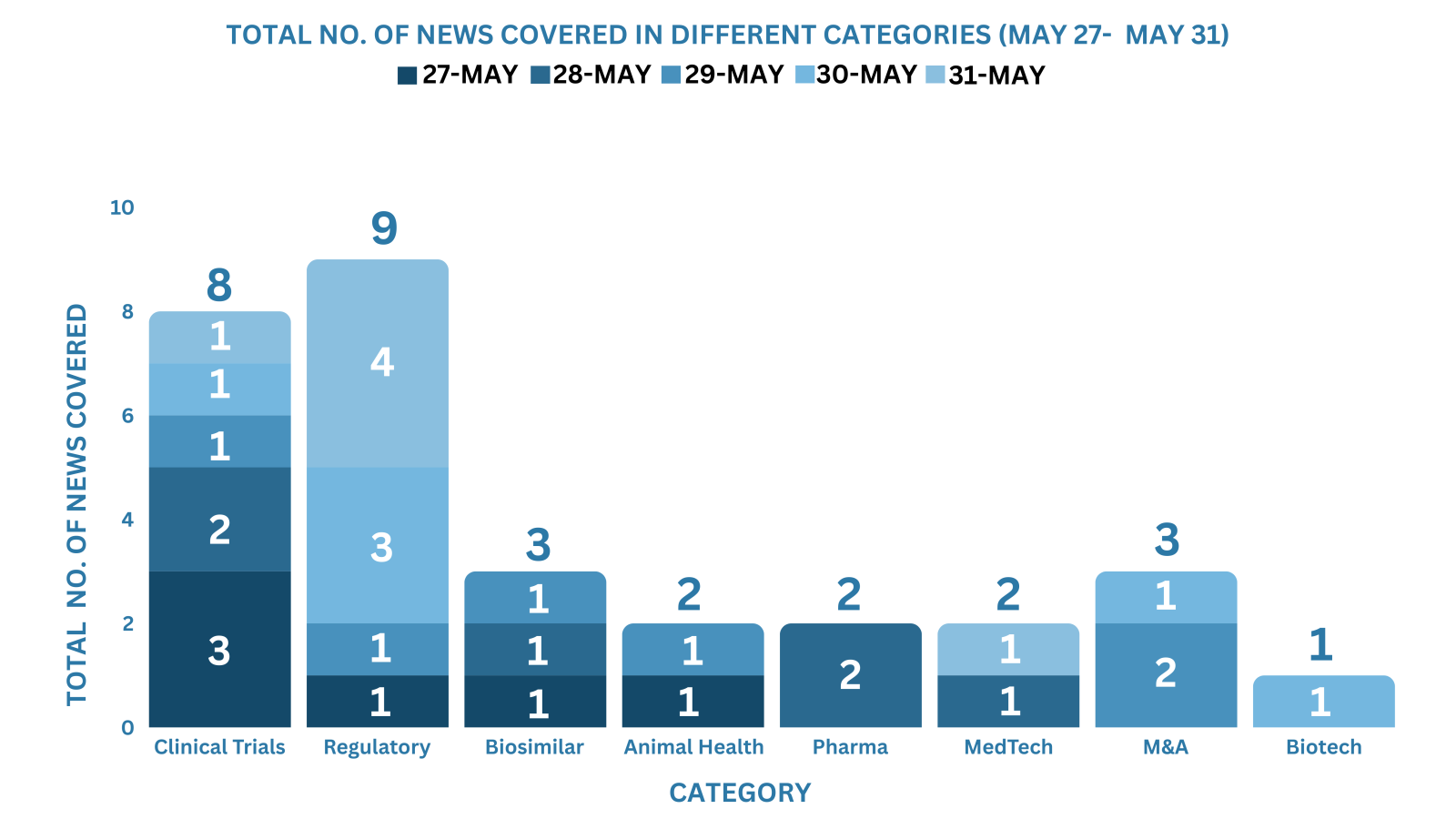
This week PharmaShots’ news was all about the updates on M&A, Pharma, Clinical Trials, Regulatory & MedTech. Check out our full report below:

AstraZeneca & Daiichi Sankyo Report the P-III (TROPION-Lung01) Study Results of Datopotamab Deruxtecan (Dato-DXd) to Treat Advanced Non-Squamous NSCLC
Read More: AstraZeneca & Daiichi Sankyo
Novartis Highlights Results from the P-III (ALIGN) Study of Atrasentan for the Treatment of IgA Nephropathy (IgAN) at ERA 2024
Read More: Novartis
CG Oncology to Highlight the P-II (CORE-001) Trial Data of Cretostimogene Grenadenorepvec Plus Keytruda for Bladder Cancer at ASCO 2024
Read More: CG Oncology
Neuren Pharmaceuticals Reports the P-II Study Results of NNZ-2591 for Pitt Hopkins Syndrome
Read More: Neuren Pharmaceuticals
Innovent Reports the P-III (CLEAR-1) Study Data of Picankibart for Treating Plaque Psoriasis in Chinese Patients
Read More: Innovent
Merck Reports the Data from P-III (KEYNOTE-522) Study of Keytruda for Treating Triple Negative Breast Cancer (TNBC)
Read More: Merck
Johnson & Johnson Reports P-III (MDD3001) Study Data of Seltorexant for Treating Major Depressive Disorder
Read More: Johnson & Johnson
Novartis Highlights the P-III (REMIX) Trial Data of Remibrutinib to Treat Chronic Spontaneous Urticaria at EAACI 2024
Read More: Novartis

Sanofi Reports the US FDA’s sBLA Acceptance of Sarclisa to Treat Transplant-Ineligible Newly Diagnosed Multiple Myeloma
Read More: Sanofi
The US FDA Accepts and Grants Priority Review to Roche’s NDA of Inavolisib for Treating Breast Cancer
Read More: Roche
BMS’ Opdivo Plus Cisplatin and Gemcitabine Gain EC’s Approval for Treating Urothelial Carcinoma
Read More: BMS
Tris Pharma’s Onyda XR Gains the US FDA’s Approval for the Treatment of Attention Deficit Hyperactivity Disorder (ADHD)
Read More: Tris Pharma
InxMed Reveals Data from the P-Ib/II Study of Ifebemtinib Plus Garsorasib for Non-Small Cell Lung Cancer (NSCLC)
Read More: InxMed
BMS Reports the US FDA’s Approval of Breyanzi for Treating Mantle Cell Lymphoma
Read More: BMS
Sanofi & Regeneron’s Dupixent Receives the CHMP’s Positive Opinion for Treating Chronic Obstructive Pulmonary Disease (COPD)
Read More: Sanofi & Regeneron
AbbVie Reports the CHMP’s Positive Opinion of Skyrizi (Risankizumab) to Treat Ulcerative Colitis
Read More: AbbVie
Eli Lilly Receives the US FDA’s Approval for Retevmo (Selpercatinib) to Treat Thyroid Cancer or Solid Tumors
Read More: Eli Lilly

The EC Grants Approval to Celltrion’s Omlyclo (Biosimilar, Xolair)
Read More: Celltrion
Fresenius Reports the US FDA’s BLA Acceptance of FKS518 (Biosimilar, Denosumab)
Read More: Fresenius
Bio-Thera Collaborates with STADA for the Commercialization of BAT2506 (Biosimilar, Simponi)
Read More: Bio-Thera & STADA

Merck Animal Health’s Innovax-ND-H5 Vaccine Gains the EC’s Marketing Authorization for Chickens
Read More: Merck Animal Health
Elanco Reports the US FDA’s Review Completion of Bovaer to be Used Across the US Dairy Industry
Read More: Elanco

Oncocross and JW Pharmaceutical Expand their Partnership to Develop New Therapies Using AI in Anticancer and Regenerative Medicine
Read More: Oncocross & JW Pharmaceutical
GlycoNex and PrecisemAb Join Hands to Develop New Anti-Glycan Antibodies for Cancer Therapy
Read More: GlycoNex & PrecisemAb

Terumo Cardiovascular Reports the US FDA’s Approval of CDI OneView Monitoring System
Read More: Terumo Cardiovascular
The US FDA Grants Clearance to InnoVoyce’s Vylo 455nm Blue Light Laser System
Read More: InnoVoyce

Johnson & Johnson to Acquire Numab's Yellow Jersey Therapeutics for an Aggregate of ~$1.25B
Read More: Johnson & Johnson & Numab
Asahi Kasei Reports the Acquisition of Calliditas Therapeutics for an Aggregate of ~$1.11B
Read More: Asahi Kasei & Calliditas Therapeutics
Merck Expands its Ophthalmologic Portfolio Through the Acquisition of EyeBio
Read More: Merck & EyeBio

Atara Biotherapeutics Reports Preclinical Data on ATA3219 to Treat B-Cell Driven Autoimmune Diseases
Read More: Atara Biotherapeutics
Related Post: PharmaShots Weekly Snapshots (May 20 – May 24, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.